Technical Data
Operating procedure (Start/ Stop)
Maintenance
Trouble
Thermal Desorption Spectroscopy
- What is the Thermal Desorption Spectroscopy
- The Thermal Desorption Spectroscopy method
- Quantitative method
- Data analysis simulation
- Acquisition data example
Photo-Stimulated Desorption
QMS
Difficult samples
Literature
Principles of the PSD method
Photo Stimulated Desorption occurs through three processes. (1 Photo-stimulation, 2 Bond cleavage, 3 Desorption)
① Photo-stimulation
Electrons in the σ orbital (bonding orbital) are excited into the σ* orbital (antibonding orbital) when they absorb light with a wavelength corresponding to the energy difference between theσ andσ* orbitals.
By using vacuum UV with a high luminous flux density, samples are excited efficiently, even thin films with short optical paths.
②Bond cleavage
When there is one electron in each of the σ andσ* orbitals, the binding energy is the sum of the two orbital levels. When the binding energy is negative, theσ bond is cleaved.
③ Desorption
Various fragments are generated when a σ bond is cleaved. For some molecules, recombination occurs on the surface. When there are generated fragments that are not contained within the sample, this is called desorption.
Next, the potential curve will be explained.
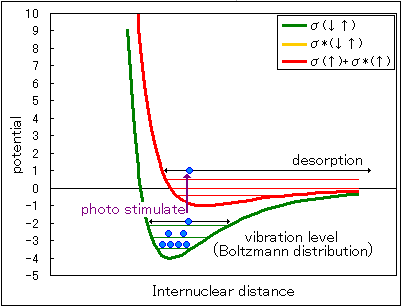
Fig1 Potential curve
In the level where two electrons are in the bonding orbital (Fig. 1 potential curve green), the same kind of bond has a Boltzmann distribution in each vibration level.
The distribution ratio of the vibrational level depends on the temperature, and the higher the temperature is, the higher the level.
When vacuum ultraviolet light of a wavelength causing an electronic transition hits this bond, one electron is excited in each of the bonding orbital and the antibonding orbital (potential curve in red in the figure above).
At this time, the bond in the vibrational level at potential energy of zero or more breaks.
(Even if the two electrons are in the bonding orbital, raising the temperature causes a transition to the vibrational level at potential energy of zero or more, causing thermal desorption.)
And when the generated fragment is not bound, the fragment is released and measured by mass spectrometer.
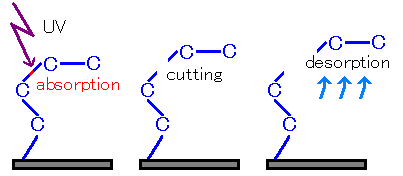
Fig2 Vacuum ultraviolet cutting
On the other hand, when multiple bonds or fragments are bound, the bond may not be completely cut, or may not be possible to detach even if it is cut.
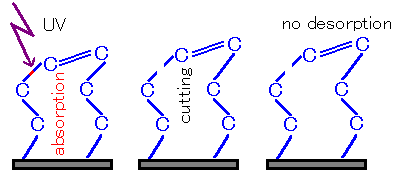
Fig3 Disconnected but not detached①
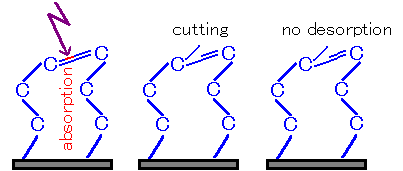
Fig4 Disconnected but not detached②
